3D Printing with PEEK, PEKK, & ULTEM™
Join AON3D's 3D printing application and materials experts for a 30-minute session that covers all the key information
Replacing metals with polymers is not only possible but becoming increasingly more prevalent. The current generation of high-performance polymers and composites has similar mechanical properties to aluminum at a fraction of the weight. In addition, the ability to additively manufacture parts from these polymers, with high temperature 3D printers like the AON Hylo, has driven further adoption by reducing the complexity, lead times, and cost constraints of traditional manufacturing methods.

What differentiates metals and polymers?
Before we address which polymers are suitable for replacing metals, we need to set the right expectations. Some companies may inflate the capabilities of high-performance polymers and fail to consider that metals and polymers are fundamentally different. Here’s how the differ:
Polymers
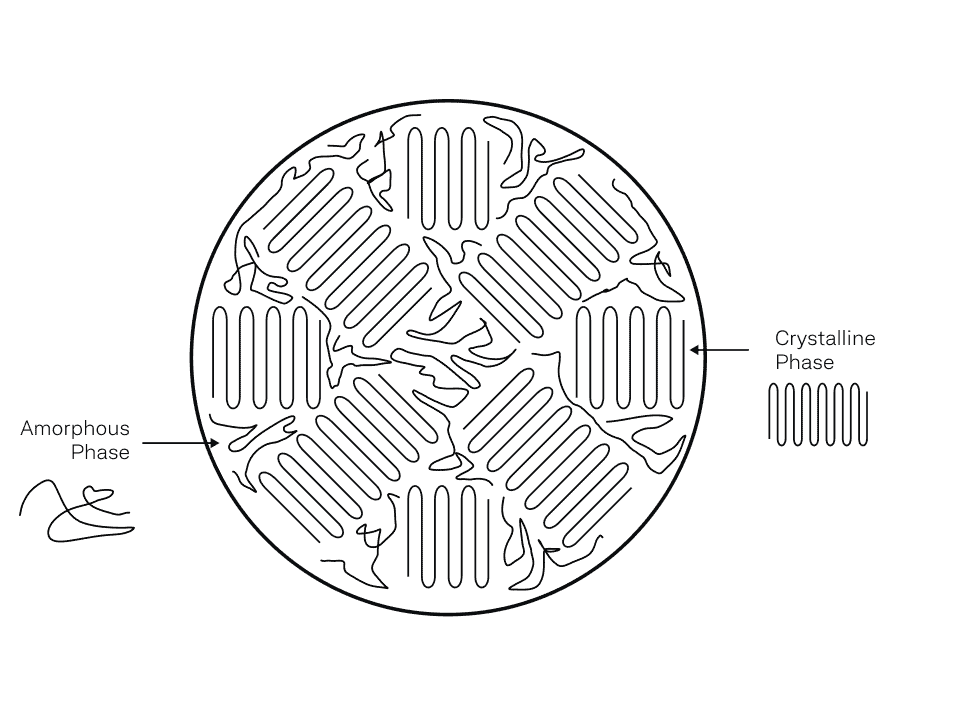
3D printable polymers are thermoplastics, made up of polymer chains that are chemically separate from each other (no molecular bonding between chains). These chains are physically entangled which provides mechanical integrity to the overall material. The chains can also organize and fold into a crystal structure which are harder to pull apart due to greater intermolecular interaction forces. This is why semi-crystalline polymers are better in strength and stiffness compared to amorphous polymers.
Metals
If you were to put a common metal under the microscope, you’d see a structure similar to image A below. Metals are made up of small grains, where each grain consists of a crystal lattice (image B). These crystals are made of an array of metal atoms that are connected through strong metallic bonding. The bonding and the organization of atoms into crystals is why metals are naturally strong and stiff. Often these grains differ from each other by the size and orientation both of which influence the bulk properties of the metal.
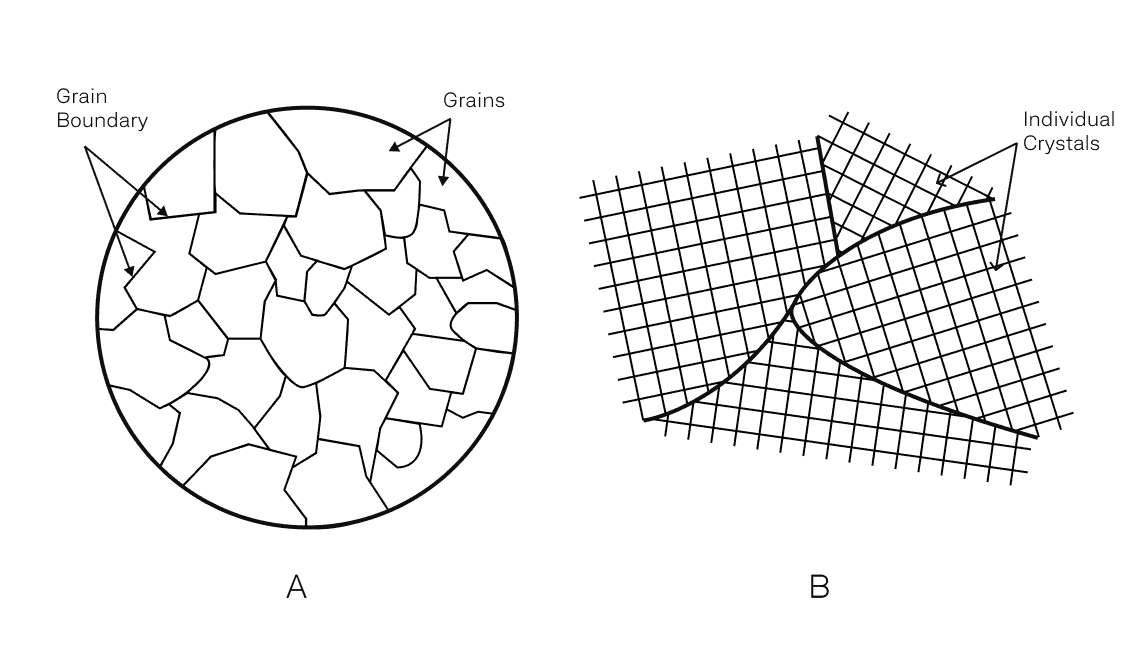
In conclusion, semi-crystalline polymers are partially made of crystals while metals contain many crystals (grains) that differ in orientation. Since thermoplastic polymer chains are chemically separate, the attraction force is weaker than the metallic bonding between crystals in metals. As a result, metals are more resistant to elastic deformation (stiffer) and generally more temperature resistant. It’s the reason why metals have been the default choice for mechanical and thermal applications. But as we engineer more advanced thermoplastics, the effective strength begins to match and, in some cases, surpass metals.
Two Suitable Metal Replacement Materials: PEEK and ULTEM™
PEEK and ULTEM™ 9085 are two of the most popular polymers that have been considered for replacing metals. The graph below compares these materials to Aluminum 6061, an alloy used in the construction of aircraft structures, automotive parts, marine structures, and consumer products. CF PEEK contains 10% carbon fiber which increases the strength and stiffness of baseline PEEK. These are several industries where metal replacement can provide significant weight savings while maintaining the required mechanical and thermal properties. In addition, these high temperature thermoplastics demonstrate superior chemical and corrosion resistance compared to common in-service metals.
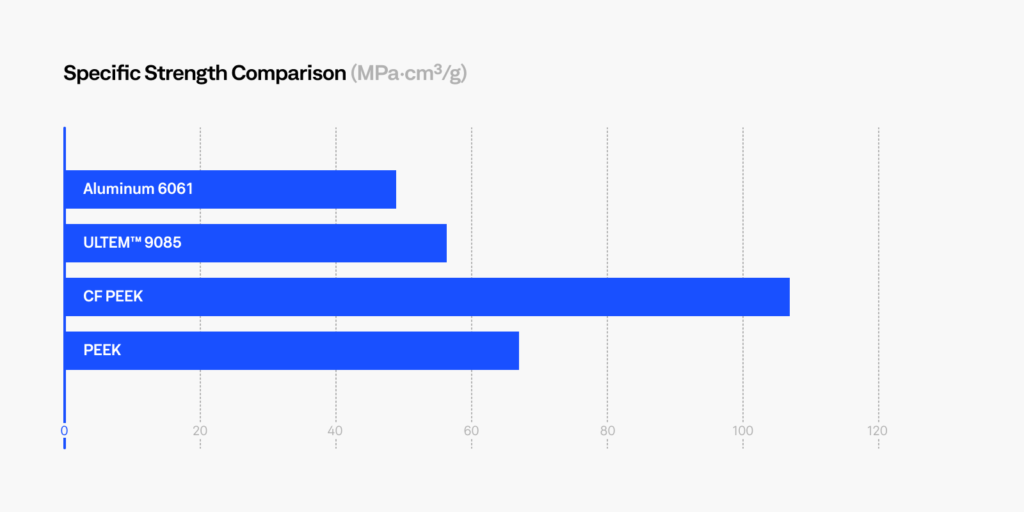
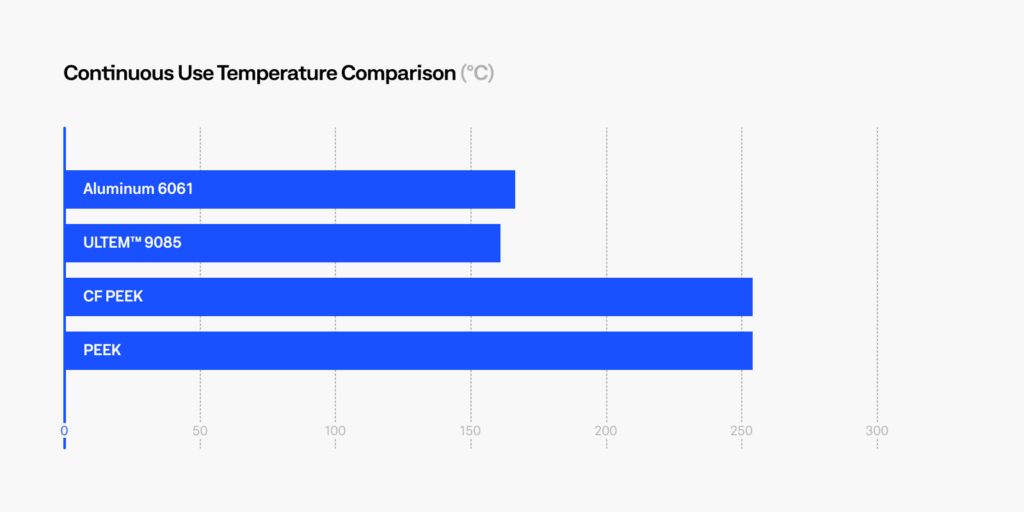
We can see that PEEK and ULTEM™ offers between 50-60% weight reduction, while maintaining high strength and high heat resistance. Replacing metals means greater fuel savings, lower carbon emissions or increased payload capabilities for the transportation industry. In addition, these materials possess high chemical resistance, high impact resistance, are UL94-V0 flammability rated, and meets FAR 25.853 flame, smoke, and toxicity (FST) requirements, a requirement in aerospace and naval applications.
How to 3D Print PEEK and ULTEM™
Simply 3D printing high performance materials won’t guarantee that the parts will possess the reported mechanical, thermal, and chemical properties. To match these properties, the selected 3D printer needs a precision-controlled high temperature build chamber (up to 260°C), 500°C extruders, and configurable print surfaces. Printing at temperatures colder than 140°C can significantly reduce part strength, chemical resistance, and require further annealing in which warping is unavoidable. AON3D Hylo is a large, high temperature industrial 3D printer optimized to make 3D printing high performance materials like PEEK and ULTEM™ 9085 easy and accessible. Click here to learn more.
Achieve New Levels of Part Performance & Throughput
3D print the world's highest performance polymers - bigger, faster, and stronger than ever.

